Ensuring Informed Consent and Patient Privacy in Genetic Testing and Analysis: Protocols in US Medical Laboratories
Summary
- Medical laboratories in the United States follow strict protocols to ensure Informed Consent and patient privacy in Genetic Testing and analysis.
- These protocols include obtaining written consent from patients before conducting Genetic Testing, ensuring confidentiality of patient information, and following guidelines set by regulatory bodies.
- By adhering to these protocols, medical laboratories can ensure that patients are fully informed about the Genetic Testing process and their privacy is protected throughout the testing and analysis.
Introduction
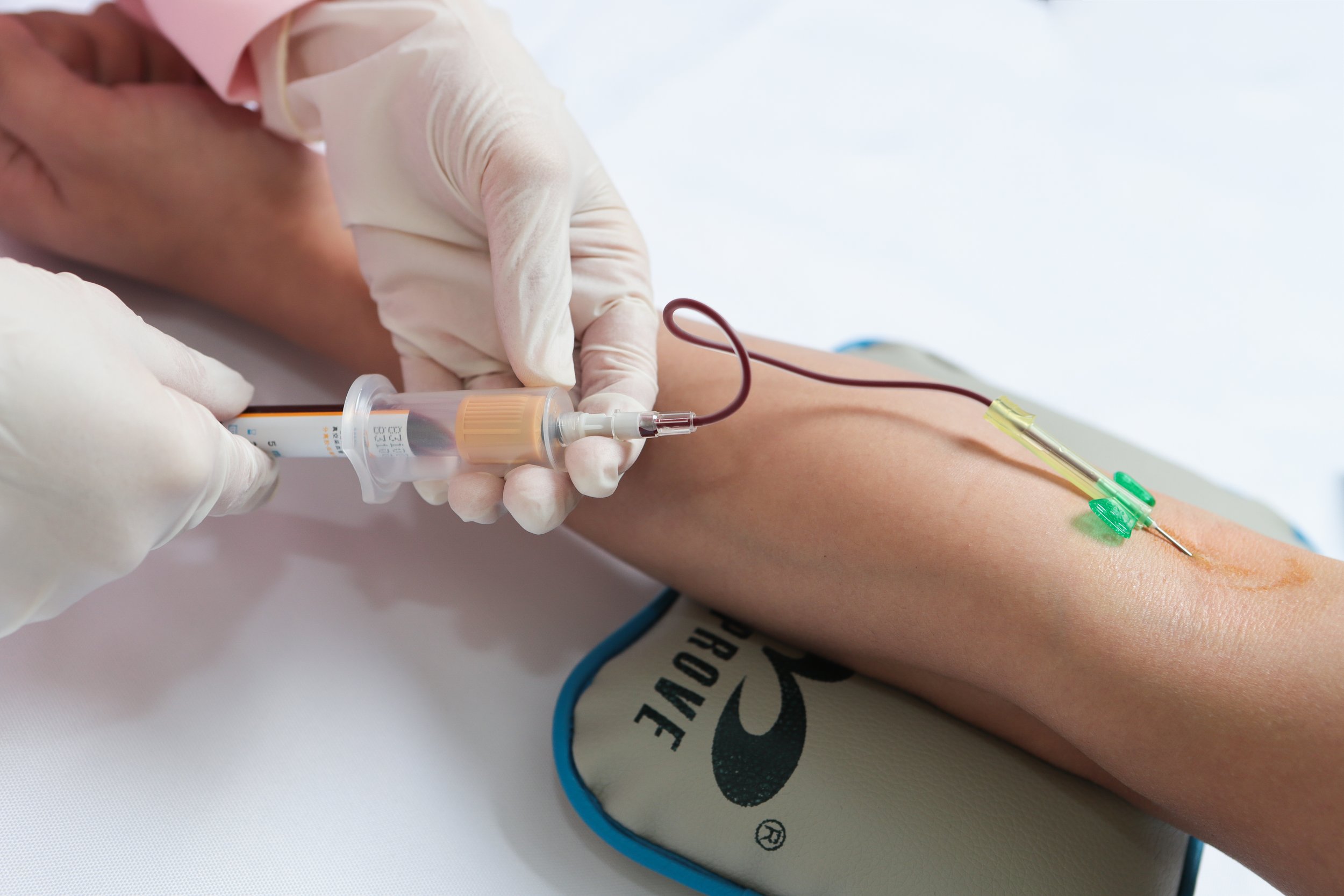
In recent years, Genetic Testing and analysis have become increasingly common in medical laboratories across the United States. These tests have the potential to provide valuable insights into a person's health risks, potential genetic disorders, and personalized treatment options. However, it is essential that patients are fully informed about the testing process and that their privacy is protected throughout the analysis. In this article, we will explore the protocols that are in place to ensure Informed Consent and patient privacy in Genetic Testing and analysis in medical laboratories.
Informed Consent
One of the key protocols in place to protect patient rights in Genetic Testing is Informed Consent. Informed Consent is the process by which patients are fully informed about the nature and purpose of the Genetic Testing, as well as the potential risks and benefits, so they can make an educated decision about whether to proceed with the test. Medical laboratories follow strict guidelines to ensure that patients provide Informed Consent before conducting any Genetic Testing.
Key Aspects of Informed Consent
- Patients are provided with information about the genetic test, including how it works, what information it will provide, and any potential risks or limitations.
- Patients are informed about how their genetic information will be used and stored, as well as who will have access to this information.
- Patients are given the opportunity to ask questions and seek clarification about the Genetic Testing process before making a decision.
Obtaining Informed Consent
Medical laboratories must obtain written consent from patients before conducting Genetic Testing. This consent form outlines the nature of the test, the risks and benefits, and how the patient's genetic information will be used. By obtaining Informed Consent, medical laboratories can ensure that patients have a clear understanding of the testing process and are able to make an informed decision about whether to proceed with the test.
Patient Privacy
Protecting patient privacy is another critical aspect of Genetic Testing and analysis in medical laboratories. Patients have the right to have their genetic information kept confidential and to know that their personal health information will not be shared without their consent. Medical laboratories follow strict protocols to ensure that patient privacy is maintained throughout the testing and analysis process.
Confidentiality of Patient Information
Medical laboratories are required to adhere to strict confidentiality protocols to protect patient information. This includes safeguarding Electronic Health Records, genetic Test Results, and any other sensitive information collected during the testing process. Laboratories must have secure systems in place to prevent unauthorized access to patient data and ensure that only authorized personnel have access to this information.
Regulatory Guidelines
Regulatory bodies, such as the Health Insurance Portability and Accountability Act (HIPAA), set guidelines for the protection of patient privacy in healthcare settings, including medical laboratories. Laboratories must comply with these Regulations to ensure that patient information is safeguarded and that patients' rights are protected. Failure to comply with these Regulations can result in severe penalties for the laboratory.
Data Security
Medical laboratories use encryption, firewalls, and other security measures to protect patient data from unauthorized access or cyberattacks. By implementing robust data security protocols, laboratories can minimize the risk of patient information being compromised and ensure that patient privacy is maintained throughout the testing and analysis process.
Conclusion
Medical laboratories in the United States follow strict protocols to ensure Informed Consent and protect patient privacy in Genetic Testing and analysis. These protocols include obtaining written consent from patients, ensuring the confidentiality of patient information, and following regulatory guidelines set by bodies such as HIPAA. By adhering to these protocols, medical laboratories can ensure that patients are fully informed about the Genetic Testing process and that their privacy is protected throughout the testing and analysis.
Disclaimer: The content provided on this blog is for informational purposes only, reflecting the personal opinions and insights of the author(s) on the topics. The information provided should not be used for diagnosing or treating a health problem or disease, and those seeking personal medical advice should consult with a licensed physician. Always seek the advice of your doctor or other qualified health provider regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. If you think you may have a medical emergency, call 911 or go to the nearest emergency room immediately. No physician-patient relationship is created by this web site or its use. No contributors to this web site make any representations, express or implied, with respect to the information provided herein or to its use. While we strive to share accurate and up-to-date information, we cannot guarantee the completeness, reliability, or accuracy of the content. The blog may also include links to external websites and resources for the convenience of our readers. Please note that linking to other sites does not imply endorsement of their content, practices, or services by us. Readers should use their discretion and judgment while exploring any external links and resources mentioned on this blog.
