New Regulations on FDA Imports for Medical Devices: Impact on Patient Care and Lab Standards
Summary
- New Regulations on FDA imports for medical devices will increase safety and Quality Standards
- Phlebotomy labs will need to adhere to stricter guidelines to ensure compliance
- The United States medical industry will see improvements in patient care and outcomes due to these Regulations
Introduction
Medical devices play a crucial role in the healthcare industry, aiding in the diagnosis, treatment, and monitoring of patients. The Food and Drug Administration (FDA) is responsible for regulating medical devices in the United States, ensuring they meet safety and efficacy standards before they can be sold on the market. With new Regulations set to impact FDA imports for medical devices, the industry is poised for significant changes.
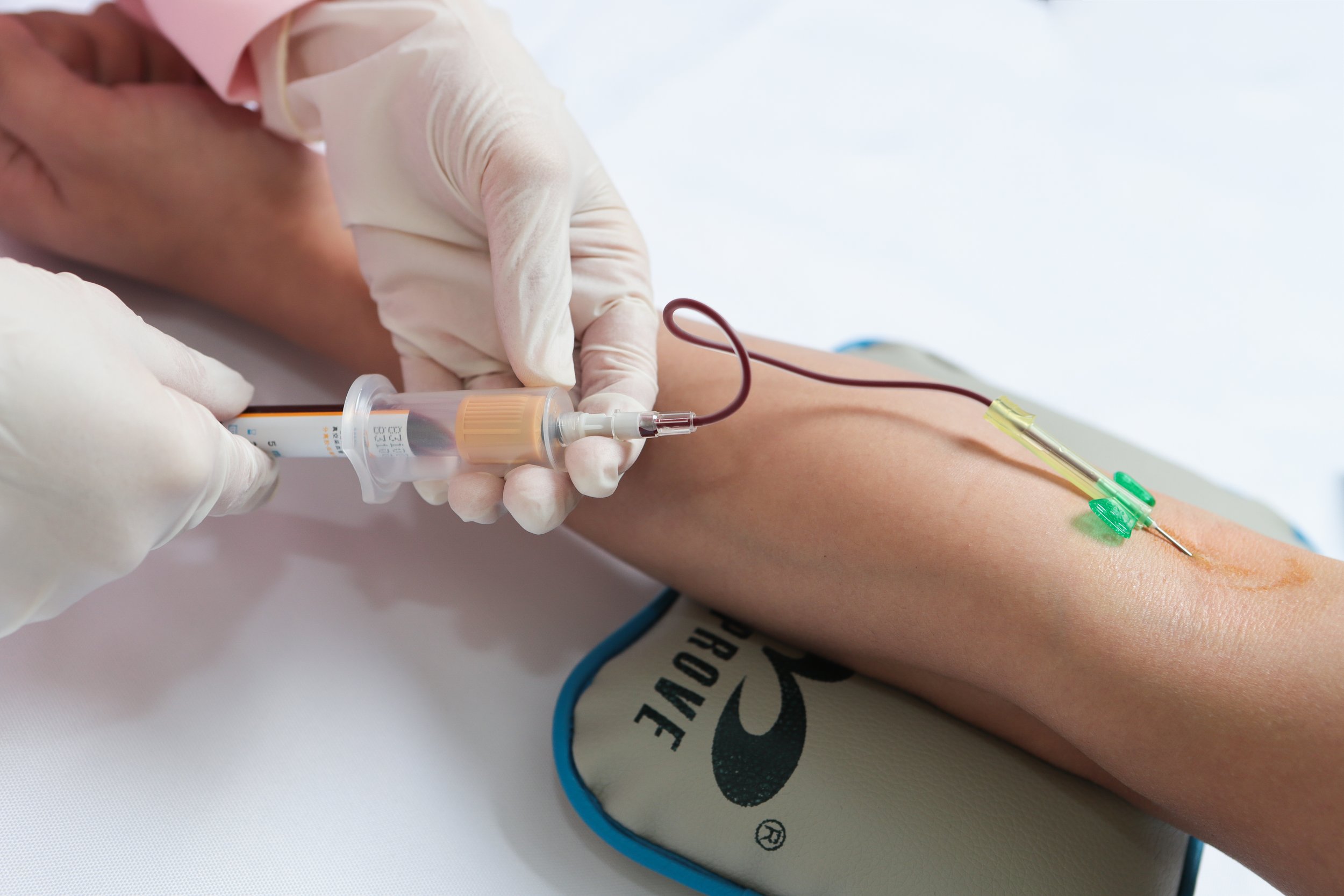
Impact on Medical Labs
Quality and Safety Standards
One of the primary impacts of the new Regulations on FDA imports for medical devices is the implementation of stricter quality and safety standards. Medical labs that rely on these devices for testing and diagnostics will need to ensure that all equipment meets these new requirements. This will ultimately benefit patients by minimizing the risk of faulty or substandard devices being used in their care.
Compliance Requirements
Phlebotomy labs, in particular, will need to pay close attention to the compliance requirements set forth by the FDA. These labs rely heavily on medical devices for drawing blood, processing samples, and conducting tests. Ensuring that all devices meet the new Regulations will be crucial to maintaining the quality and accuracy of their services.
Training and Education
With the introduction of new Regulations, medical lab technicians and phlebotomists may need additional training and education to stay informed on the changes. Understanding how the Regulations impact their work and what steps need to be taken to comply will be essential for providing quality care to patients.
Impact on Patient Care
Improved Outcomes
By raising the standards for FDA imports of medical devices, patients can expect to see improved outcomes in their care. With higher quality and safer equipment being used in medical labs, the accuracy of tests and diagnostics will increase, leading to more accurate diagnoses and better treatment plans.
Enhanced Safety
Patient safety is a top priority in the healthcare industry, and the new Regulations on FDA imports for medical devices will further enhance safety measures. By ensuring that only safe and effective devices are used in medical labs, the risk of adverse events and errors will be minimized, ultimately benefiting patients and Healthcare Providers alike.
Increased Trust
As medical labs adhere to the new Regulations and maintain compliance with FDA import standards, patients can place their trust in the quality of care they receive. Knowing that their tests and treatments are being conducted with safe and reliable equipment will instill confidence in the healthcare system and promote better patient-provider relationships.
Conclusion
The new Regulations on FDA imports for medical devices will have a significant impact on the medical lab and phlebotomy industry in the United States. By raising quality and safety standards, improving compliance requirements, and enhancing training and education, these Regulations will ultimately lead to better patient care and outcomes. As the industry adapts to these changes, the focus on safety, quality, and efficacy will continue to drive improvements in healthcare delivery.
Disclaimer: The content provided on this blog is for informational purposes only, reflecting the personal opinions and insights of the author(s) on the topics. The information provided should not be used for diagnosing or treating a health problem or disease, and those seeking personal medical advice should consult with a licensed physician. Always seek the advice of your doctor or other qualified health provider regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. If you think you may have a medical emergency, call 911 or go to the nearest emergency room immediately. No physician-patient relationship is created by this web site or its use. No contributors to this web site make any representations, express or implied, with respect to the information provided herein or to its use. While we strive to share accurate and up-to-date information, we cannot guarantee the completeness, reliability, or accuracy of the content. The blog may also include links to external websites and resources for the convenience of our readers. Please note that linking to other sites does not imply endorsement of their content, practices, or services by us. Readers should use their discretion and judgment while exploring any external links and resources mentioned on this blog.
